Four monomeric copper(ii) complexes of non-steroidal anti-inflammatory drug Ibuprofen and N-donor ligands: syntheses, characterization, crystal structures and cytotoxicity studies†
Abstract
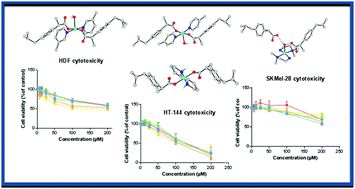
Reaction of hydrated copper(II) ibuprofenate with various nitrogen donor ligands, β-picoline, γ-picoline, pyrrolidine and unsymetrical dimethyl ethylenediamine (unsym-dmen), at room temperature in a methanol : water mixture (4 : 1 v/v) yielded four new complexes, [Cu(Ibu)2(β-picoline)2(H2O)], 1; [Cu(Ibu)2(γ-picoline)2(H2O)]·H2O, 2; [Cu(Ibu)2(pyrrolidine)2]·H2O, 3; and [Cu(unsym-dmen)2(H2O)](Ibu)2, 4, respectively, where Ibu = deprotonated Ibuprofen (HIbu). The newly synthesized complexes have been characterized by elemental analyses, spectroscopic methods (FT-IR, UV-Vis and EPR), TGA and single crystal X-ray structure determination. Crystallographic investigations revealed that all the complexes are monomeric in nature, in contrast to the dimeric nature of copper(II) ibuprofenate solvates. EPR studies clearly revealed that the chromophore present in all complexes (1–4) is consistent with the structures determined by X-ray crystallography. The cytotoxic effects of all complexes were tested by a colorimetric assay on three human cell lines; though the activity was affected by the nature of the cell line, the newly synthesized complexes 1–4 showed higher cytotoxicity than the parent molecule against all the tumoral cell lines.


 Please wait while we load your content...
Please wait while we load your content...