‘One-pot’ synthesis and redox evaluations of chiral chalcogenocysteinol and β-bis-chalcogenoamine derivatives from l-serine methyl ester†
Abstract
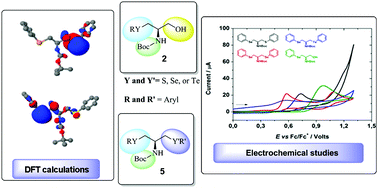
The synthesis of a new class of chiral chalcogenocysteinols and bis-chalcogenoamines is described in this study. The compounds were prepared from commercially available L-serine using simple reactions to obtain the desired products. Additionally, the compounds were evaluated for potential antioxidant applications by cyclic voltammetry and shown to have an appropriate electrochemical oxidation potential. Density functional theory (DFT) calculations were used to better characterize the oxidative sites in the bis-chalcogenoamines, giving theoretical support to the experimental findings.


 Please wait while we load your content...
Please wait while we load your content...