Superhalogens as building blocks of a new series of superacids
Abstract
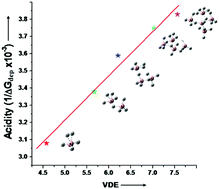
Strong superacids, such as HSbF6, typically consist of Brønsted/Lewis acid components (e.g., HF/SbF5). Using MP2/6-311++G(d,p) calculations, we propose a new series of superacids by the protonation of BnH3n+1− superhalogen anions. The resulting BnH3n+2 species behave as superacids for n ≥ 2 due to their smaller free energy of deprotonation (ΔGdep = 292.7–258.8 kcal mol−1) than that of H2SO4 (ΔGdep = 302.2 kcal mol−1). These BnH3n+2 superacids do not require Brønsted/Lewis acid components, whose gas phase acidity is governed by the vertical detachment energy (VDE) of the BnH3n+1− superhalogens. The larger VDEs of BnH3n+1− allow the BnH3n+2 species to easily release H+, increasing their acidic strength. We have also investigated other superhalogens, such as HSO4−, BO2− and ClO4−, and noticed that their VDEs follow the same order as that of the gas phase acidities of their protonated species, namely HClO4 > H2SO4 > HBO2.


 Please wait while we load your content...
Please wait while we load your content...