Efficient mass-fabrication of amorphous MnSiO3/C with high stability through a simple water-boiling treatment and its Li-ion storage performance†
Abstract
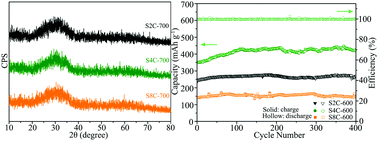
Amorphous manganese silicate was fabricated by simply heating a mixture of Na2SiO3·9H2O and MnCl2·4H2O in boiling water (called a water-boiling treatment). Even after being coated with carbon at 700 °C, employing glucose as the carbon source, the silicate is still in the amorphous state. The electrochemical performance of the carbon-coated amorphous silicate as an anode material for Li-ion batteries was evaluated. The carbon-coated product with a water-boiling time of 4 h exhibits excellent rate performance and high-rate cyclability. The amorphous silicate with a porous structure and a large specific surface area is beneficial to Li-ion transportation, and the homogeneous carbon coating around the silicate nanoparticles as a conductive material is conducive to improving their electronic conductivity.


 Please wait while we load your content...
Please wait while we load your content...