Hexaaryl-carbo-benzenes revisited: a novel synthetic route, crystallographic data, and prospects of electrochemical behavior†
Abstract
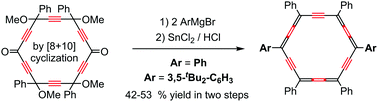
An improved 12-step synthetic route and full characterization of hexaphenyl-carbo-benzene (4, 8%) and the p-bis-3,5-di-tert-butylphenyl homologue (11, 4%), are described. The carbo-benzene reference 4 is now accurately described in the crystal state by X-ray diffraction analysis in the chiral space group P212121, and in comparison to the less symmetric derivative 11 exhibiting a centro-symmetric packing. According to cyclic voltammetry, both hexaaryl-carbo-benzenes 4 and 11 can behave as both reversible potent electron acceptors and standard electron donors, with respective potentials of −0.73 ± 1 V and +1.17 ± 2 V/SCE, respectively. Due to their extremely low solubility, solid films of 11 fabricated using the “wet method”, with the initial view of studying charge transport properties, were found to display high roughness.


 Please wait while we load your content...
Please wait while we load your content...