Synthesis, photophysics and electronic structure of oxobacteriochlorins†
Abstract
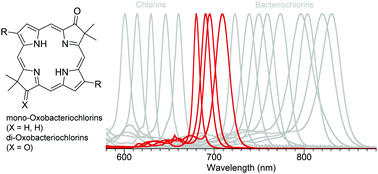
Synthetic routes are described to access mono- and dioxobacteriochlorins that lack any peripheral substituents, with the exception of a geminal dimethyl group in each pyrroline ring to stabilize the macrocycle towards adventitious oxidation. The introduction of one or two oxo groups on the pyrroline rings results in hypsochromic shifts of the longest wavelength near-infrared absorption band (Qy) to 690 and 680 nm, respectively, versus the parent bacteriochlorin for which the Qy band is at 713 nm. The position of the Qy absorption band of the oxobacteriochlorin is in the range of that of elaborately functionalized chlorin macrocycles (containing one pyrroline ring versus two in a bacteriochlorin), where the functional groups bathochromically shift the Qy feature from that of unfunctionalized chlorins (typically in the low 600 nm region). The long-wavelength absorption band of synthetic oxobacteriochlorins thus appears in the window defined by simple chlorins to the red and substituted bacteriochlorins to the near-infrared. The ability to access the deep-red (680–690 nm) region via the relatively simple synthetic modification of oxo-group addition to a bacteriochlorin versus the more complex multifunctionalization of a chlorin is attractive for accessing tetrapyrroles for solar-energy conversion and other applications.


 Please wait while we load your content...
Please wait while we load your content...