Highly active Ni–Fe double hydroxides as anode catalysts for electrooxidation of urea†
Abstract
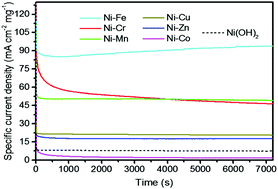
Urea is a safe and sustainable chemical for electrochemical energy conversion and storage. However, the sluggish kinetic rates of the electrooxidation reaction of urea as well as catalyst stability still remain to be challenges. In this work, we investigated several catalysts for the electro-oxidation of urea by directly growing NiM double hydroxides (M = Cr, Mn, Fe, Co, Cu, Zn) on carbon fibre cloth and nickel foam electrodes through a facile one-step hydrothermal synthesis method. The results indicated that the activity was significantly related to the elemental composition. Among the investigated double hydroxides, the NiFe double hydroxide (DH) catalyst showed the highest activity, which achieved a specific current density of ∼95 mA cm−2 mg−1 at 0.5 V vs. Ag/AgCl, about 10 times larger than that of Ni(OH)2. In addition, the NiFe DH also had a high activity when grown on a Ni foam substrate. This NiFe DH performs well in the aspect of urea oxidation stability, demonstrating it to be a promising low-cost and stable catalyst for the efficient electrooxidation of urea under alkaline conditions.


 Please wait while we load your content...
Please wait while we load your content...