A synthetic route towards 3,4-disubstituted pyrrolidin-2-ones via a Michael addition and reductive ring closing strategy†
Abstract
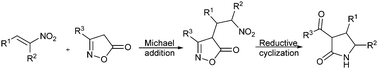
Pyrrolidin-2-one derivatives were synthesized via DABCO mediated Michael addition of isoxazol-5(4H)-ones with nitroalkenes, followed by one pot reduction of the nitro group and ring cleavage with cyclization. 2-Pyrrolidinone scaffolds with a wide range of substituents were synthesized with good yield and diastereoselectivity by using this protocol.


 Please wait while we load your content...
Please wait while we load your content...