Cycling stability of the Li3(V0.9Mg0.1)2(PO4)3/C cathode with an increase in the charge cut-off voltage
Abstract
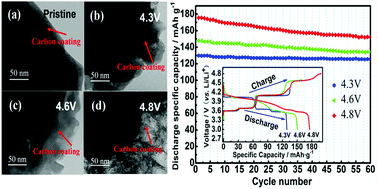
The problem of the decrease in cycling stability of Li3(V0.9Mg0.1)2(PO4)3/C limits its practical application in a broad electrochemical window. However, the cause of the cycling stability decrease in Li3(V0.9Mg0.1)2(PO4)3/C has not been studied in previous research. In this paper, to illustrate the cause of the decreased cycling stability of the Li3(V0.9Mg0.1)2(PO4)3/C samples, we investigated the crystal structure changes in the cathode materials in a broad electrochemical window. The structure of the Li3(V0.9Mg0.1)2(PO4)3/C samples was analyzed by XRD refinement, SEM and TEM. The results indicate that the higher the charge cut-off voltage is, the worse the cycling stability of the sample is. It was concluded that the cell volume of the Li3(V0.9Mg0.1)2(PO4)3/C samples expands irreversibly after cycling in different voltage ranges, and the bond lengths of Li(3)–O become longer while those of Li(2)–O and Li(1)–O become shorter. This means that the bond energy of the Li(3) ion increased, and the bond energy of the Li(1) and Li(2) ions decreased. This is not beneficial to the intercalation/deintercalation of Li ions with the increase in the charge cut-off voltage. The TEM test shows that the carbon layer of the samples is destroyed with the increase in the charge cut-off voltage. It is reasonably inferred that the crystal structure change in the Li3(V0.9Mg0.1)2(PO4)3/C samples causes poor cycling stability with the increase in the charge cut-off voltage.


 Please wait while we load your content...
Please wait while we load your content...