Synthesis and antiproliferative activity of sulfa-Michael adducts and thiochromenes derived from carbohydrates†
Abstract
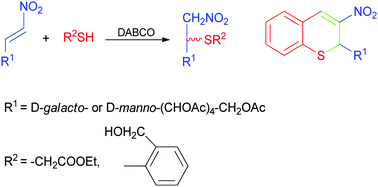
The Michael addition reactions of carbohydrate-derived nitroalkenes with ethyl thioglycolate and 2-mercaptobenzyl alcohol were studied. Reactions were conducted under mild, solvent-free conditions with DABCO as a catalyst, affording the corresponding adducts in good yields. Furthermore, compounds resulting from the addition with 2-mercaptobenzyl alcohol were used as starting materials for the synthesis of chiral 3-nitro-2H-thiochromenes. For some of the compounds synthesized herein, the antioxidant and antiproliferative activities against a panel of human solid tumor cell lines were assayed and compared with those of carbohydrate-nitroalkene substrates.


 Please wait while we load your content...
Please wait while we load your content...