Diglycolamic acid-functionalized multiwalled carbon nanotubes as a highly efficient sorbent for f-block elements: experimental and theoretical investigations
Abstract
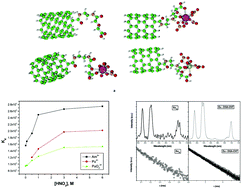
Diglycolamic acid-functionalized carbon nanotubes were employed for the efficient and selective separation of Pu4+, PuO22+ and Am3+. The sorption occurred through monolayer coverage via chemisorption following the Langmuir isotherm. The sorption of Am3+, Pu4+ and PuO22+ on DGA-CNTs proceeded via a pseudo-second-order rate kinetics with a rate constant in the order Am3+ < Pu4+ < PuO22+. The radiolytic stability for DGA-CNTs and the stripping behaviour of DGA-CNTs were investigated. Luminescence investigations revealed the presence of single species without an inner-sphere water molecule. On complexation, the asymmetry around Eu3+ was found to be enhanced along with the covalency of the metal–ligand bond. Additionally, DFT calculations were performed to investigate the coordination and complexation interaction behaviour of CNT-DGA towards the metal ions. The present DFT study revealed a tridentate coordination mode of the DGA moiety towards Pu4+ and Am3+ and a bidentate coordination mode towards PuO22+. The calculated binding energy of sorption with DGA-CNTs towards Pu4+ was found to be higher than that of PuO22+, in both the gaseous and aqueous phase, whereas for Am3+, it was higher than that for PuO22+ but less than that for Pu4+. The free energy of sorption was also found to be highest for Pu4+ uptake and lowest for PuO22+, in both the gaseous and aqueous phase.


 Please wait while we load your content...
Please wait while we load your content...