Investigation of the active sites for NO oxidation reactions over MnOx–CeO2 catalysts†
Abstract
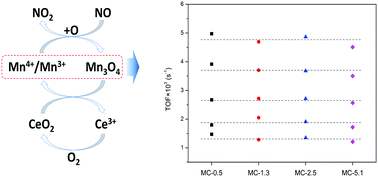
MnOx–CeO2 is a potential substitute for commercial Pt-based diesel oxidation catalysts. To better investigate its active sites for NO oxidation reactions, a series of MnOx–CeO2 samples with low Mn doping amounts (≤5%) were synthesized using the incipient-wetness impregnation method. The catalysts were characterized by various techniques (XRD, BET, XPS and H2-TPR). According to XRD and XPS, a trace amount of manganese enriched on the surface of CeO2 exists in higher oxidation states. With the increase of Mn doping, more Mn2+ is introduced into the CeO2 lattice. The interaction between the manganese oxides and ceria improves the redox properties of MnOx and the surface oxygen of CeO2. Combining turn over frequency with the initial reducibility from H2-TPR quantitation, it is found that H2 consumption from Mn4+/Mn3+ to Mn3O4 is equivalent to the active site for NO oxidation reactions. In conclusion, Mn4+/Mn3+ is the active species for NO oxidation reactions.


 Please wait while we load your content...
Please wait while we load your content...