Kinetics of the hydrogen evolution reaction on a highly porous three-dimensional Ni catalyst in the presence of a Mo ion activator in alkaline solution
Abstract
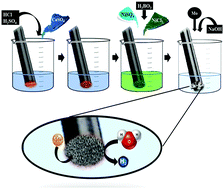
In this study, a highly porous three-dimensional structure of Ni (3D-Ni) catalyst was prepared by the dynamic hydrogen bubble template (DHBT) method. Also, its kinetics toward the hydrogen evolution reaction (HER) was studied in the absence and presence of Mo ions in 1 M NaOH acting as an in situ activator agent. Furthermore, the effect of temperature on the HER electrocatalytic activity and mechanism of the 3D-Ni catalyst was studied in the presence of 75 ppm Mo ions in electrolyte solution as the most effective concentration. The electrochemical efficiency and stability of the catalyst was evaluated on the basis of electrochemical data obtained from linear sweep voltammetry (LSV), steady-state polarization Tafel curves, electrochemical impedance spectroscopy (EIS) and chronoamperometry (CA) methods. Measurements were performed at several overpotentials (100 to 300 mV) and temperatures (298 to 333 K). Results revealed that the in situ activation of the 3D-Ni catalyst significantly increased its electrocatalytic activity towards the HER. Also, the source of its high activity arose from a high surface area and high synergetic effect. The 3D-Ni catalyst in the presence of 75 ppm Mo ions as the best condition was characterized by the Tafel slope (−189 mV dec−1), exchange current density (−18.5 × 10−3 A cm−2), overpotential at the current density of 250 mA cm−2 (−291 mV), charge transfer resistance (2.58 Ω cm2) and double layer capacitance (0.053 F cm−2) at 298 K. An important result of this work is that the high electrocatalytic activity of in situ activated 3D-Ni catalyst with Mo ions towards the HER originated from increases in surface roughness (≈75%) and synergetic effect (≈25%).


 Please wait while we load your content...
Please wait while we load your content...