Chiral phosphorescent probes for amino acids: hybrids of iridium(iii) complexes with synthetic saponite†
Abstract
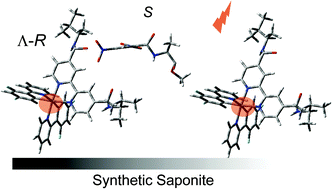
An attempt of developing a chiral luminescent probe for amino acids was described. Two types of chiral iridium(III) complexes were synthesized: [Ir(dfppy)2(R-pep-bpy)]+ (dfppyH = 2-(2′,4′-difluorophenyl)pyridine); R-pep-bpy = 4,4′-bis((R-1,2-dimethylpropyl)aminocarbonyl-2,2′-bipyridine) and [Ir(piq)2(R-pep-bpy)]+ (piqH = 1-phenyisoquinoline). In both complexes, amido groups (R-pep-) were attached to 2,2′-bipyridine with an intension of increasing the affinity for an amino acid. The complexes were optically resolved on a chiral column. When the complexes were adsorbed by the colloidal particles of synthetic saponite, they were highly emissive even in a medium containing water. The remarkable quenching of emission was observed for the combination of [Ir(dfppy)2(R-pep-bpy)]+ and tryptophan methyl ester. By the use of the enantiomeric complexes, the possibility of enantioselective quenching was examined for various amino acid derivatives.


 Please wait while we load your content...
Please wait while we load your content...