Characterization of aggregated morphologies derived from mono- and bis-arylbenzamides – potential alpha-helix mimetics†
Abstract
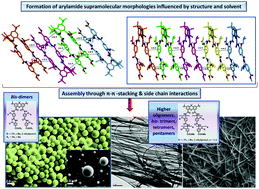
We report here the synthesis and self-assembly studies of a family of benzamide backbone oligomers bearing various alkyl side chains (e.g., isopropyl, isobutyl, and 2-ethylpentyl), which are potential alpha-helix mimetics capable of disrupting protein–protein interactions. Electron microscopy data (i.e., SEM and TEM concentration series) are indicative of the formation of various aggregates, such as micro- and nanofibers, and spherical beads, which are dominated by bis-oligoamide structures and may have resulted from intermolecular H-bonding, π–π stacking, and amide group dipole electrostatic attraction as evidenced by single crystal X-ray analysis. Thus, the aggregation behaviour was shown to depend on the number of repeat units in the oligoamide scaffold featuring elongated aggregates for bis-tetramers, whereas bis-dimers tend to form microspheres in a wide range of concentrations examined. We hypothesize that higher oligomers possessing an extended arylamide backbone are prone to efficiently crystallize with one another by interdigitation of their alkyl side chains leading predominantly to rod-like morphologies and fibrous crystals. The structural findings presented here can be potentially used in the rational design of supramolecular architectures based on arylamide peptidomimetics.


 Please wait while we load your content...
Please wait while we load your content...