Unprecedented β-manno type thiodisaccharides with a C-glycosylic function by photoinitiated hydrothiolation of 1-C-substituted glycals†
Abstract
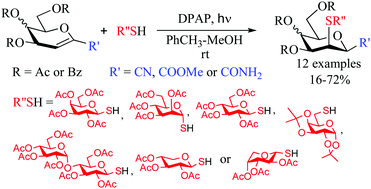
Free-radical hydrothiolation of O-peracylated 1-C-(carbamoyl-, methoxycarbonyl- and cyano) substituted glycals with a range of sugar derived thiols gave the corresponding β-manno type 3-deoxy-3-S-disaccharides with full regio- and stereoselectivity. The configuration of the glycals (arabino vs. lyxo) and the size of the protecting groups had no significant effect on the outcome of the transformations. Formation of by-products was tracked down by LCMS studies and correlated with the electron density of the double bonds to show that the reactions were synthetically useful with a COOMe and especially with a CONH2 group as the 1-C-substituent.


 Please wait while we load your content...
Please wait while we load your content...