Kinetic evidence for the formation of diazo ethers in the course of reactions between arenediazonium ions and antioxidants
Abstract
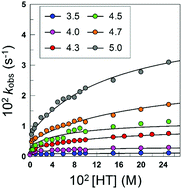
The reactions between arenediazonium ions, ArN2+, and phenolic antioxidants, AOs, are of interest because ArN2+ are involved in carcinogenic and mutagenic processes in contrast to phenolic AOs, that have biological activity and potential beneficial effects on health, and because an ArN2+ is currently being employed as a chemical probe to assess the distribution of AOs in lipid-based food emulsions. Here we have investigated the kinetics and mechanism of the reaction between 3-methylbenzenediazonium ions, 3MBD, and the antioxidant hydroxytyrosol (HT). In the absence of HT, 3MBD decompose spontaneously to an extremely reactive aryl cation that reacts with nucleophiles present in its solvation shell (DN + AN mechanism). In the presence of HT, at constant acidity, the variations of the observed rate constant, kobs, with [HT] follow saturation kinetic patterns with nonzero, pH-dependent intercepts. At constant [HT], the variation of kobs with acidity follows an upward curve suggesting that the reaction takes place with the anionic form of HT. The main mechanism in the presence of HT comprises two consecutive equilibrium steps. The first one leads to the formation of unstable (Z)-diazo ether that isomerizes to the more stable (E)-isomer. Kinetic analyses of the results allow estimations of the equilibrium constants of both equilibrium steps; the first equilibrium constant is much higher than the second.


 Please wait while we load your content...
Please wait while we load your content...