Synthesis and structural characterization of electrochemically reversible bisferrocenes containing bis(acyl-thiourea)s: enantiomers and conformers†
Abstract
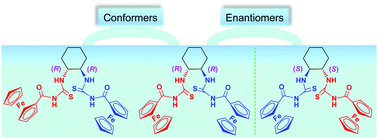
Two chiral bisferrocenyl-modified bis(acyl-thiourea) enantiomers, (1R,2R)-bis(ferrocenylcarbonylthioureido)cyclohexane (1) and (1S,2S)-bis(ferrocenylcarbonylthioureido)cyclohexane (2), were synthesized by the reactions of 2.2 equivalents of ferrocenoyl isothiocyanate with (1R,2R) and (1S,2S)-1,2-diaminocyclohexane (1,2-DACH) via a nucleophilic addition reaction, respectively. The two new compounds were fully characterized by 1H NMR, 13C NMR, IR, UV-Vis, elemental analyses and single-crystal X-ray diffraction. The cyclic voltammetry (CV) and differential pulse voltammetry (DPV) experiments of compounds 1 and 2 showed roughly similar single reversible redox waves when using nBu4NClO4 (TBAP) as the supporting electrolyte, whereas two CV and DPV waves were observed when using nBu4NBArF4 [ArF4 = 3,5-bis(trifluoromethyl)phenyl]. Furthermore, both compounds 1 and 2 displayed potential antitumor activity against human HepG2 cells. When compounds 1 and 2 were crystallized from diethyl ether, the solvent-free enantiomers (1 and 2) were obtained, respectively. Molecules of both solvent-free enantiomers (1 and 2) assemble into a three-dimensional network structure through hydrogen-bonding and C–H⋯π (cyclopentadienyl rings) interactions. On the other hand, crystallization of compound 1 from benzene produced a benzene disolvate, 1·(C6H6)2. Molecules of the solvent-free and disolvated forms of compound 1 exhibit different molecular conformations and packing arrangements.


 Please wait while we load your content...
Please wait while we load your content...