C1 and Cs 2-pyridylethylanilido zirconium(iv), yttrium(iii) and lutetium(iii) complexes: synthesis, characterization and catalytic activity in the isoprene polymerization†
Abstract
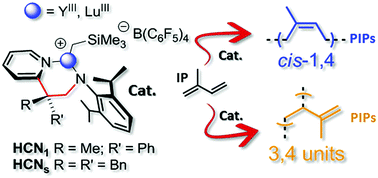
Neutral group-IV and rare-earth complexes stabilized by novel Cs and C1-symmetric 2-pyridylethylanilido ligands have been prepared and fully characterized before being scrutinized as catalyst precursors in the isoprene (IP) polymerization. In all the isolated complexes, these ligands coordinate to the metal centers in their monoanionic bidentate form. Tetra-amido ZrIV-complexes from this series (11 and 12) have shown only negligible catalytic activity in the IP polymerization, giving polydienes in traces, irrespective of the activator(s) and reaction conditions used. On the other hand, ternary systems made of a bis-alkyl rare-earth metal complex (13–16), an organoborate and a 10-fold excess of an aluminum-alkyl [pre-catalyst/Al-alkyl/borate = 1 : 10 : 1] are found to initiate the living IP polymerization with complete monomer conversion within a few minutes. The process selectivity has been investigated from different perspectives, analyzing its dependence from the rare-earth metal ion of choice (YIIIvs. LuIII), the ligand type (C1vs. Cs) and the activator(s). Polyisoprenes (PIPs) with a prevalent cis-1,4-motif (up to 67.0%) or mainly featured by vinyl pendant arms in their microstructure (up to 75.7% – 3,4-motif) are obtained.


 Please wait while we load your content...
Please wait while we load your content...