An efficient methodology to introduce o-(aminomethyl)phenyl-boronic acids into peptides: alkylation of secondary amines†
Abstract
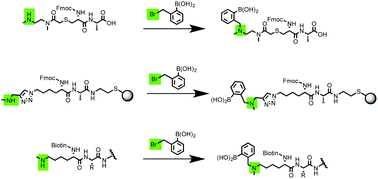
Current approaches for incorporating boronic acids into peptides require one of the following: the synthesis of commercially unavailable pinacol-protected boronate ester amino acid building blocks, amidation of small-molecule amine-containing boronic acids, or reductive amination of amine residues with 2-formylphenyl boronic acid. These methods have drawbacks, such as the use of excess starting materials, the lack of reactive-site specificity, or the inability to add multiple boronic acids in solution. In addition, several of these approaches do not allow for incorporation of the critical o-aminomethyl functionality that allows for binding of saccharides under physiological conditions. In this work, we report three methods to functionalize synthetic peptides with boronic acids using solid-phase and solution-phase chemistries by alkylating a secondary amine with o-(bromomethyl)phenylboronic acid. Solution-phase chemistries afforded the highest yields, and were used to synthesize seven complex biotinylated multi-boronic acid peptides.

 Please wait while we load your content...
Please wait while we load your content...