Which fullerenols are water soluble? Systematic atomistic investigation†
Abstract
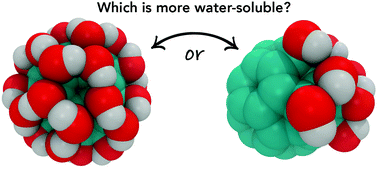
Fullerenols are hydroxylated fullerenes, C60(OH)n, where n can be up to 40 depending on the method of synthesis. Fullerenols are considered for biomedical applications, thanks to their antioxidant activities. Aqueous solubilities of fullerenols are essential to efficiently deliver them to living bodies. We hereby report the hydration thermodynamics of fullerenols C60(OH)8, C60(OH)16, C60(OH)24, C60(OH)36, and C60(OH)44 and correlate them to the solute–solvent hydrogen bonding using classical molecular dynamics simulations. Two types of hydrogen bonds were detected: O(hydroxyl)–H(water) and H(hydroxyl)–O(water), whose parameters are well comparable. The most favorable hydration free energy was observed for C60(OH)36, whereas the hydration of C60(OH)44 is clearly less efficient, in spite of a larger number of hydroxyl groups. The beneficial action of each hydroxyl group decreases with their quantity within the fullerenol molecule increases. Hydrogen bonds were characterized (life times, geometries, and thermodynamics) in accordance with the Luzar–Chandler theory. Our systematic analysis of fullerenols identified the most suitable compositions to be used in the emerging biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...