Reversible piezofluorochromism of a triphenylamine-based benzothiazole derivative with a strong fluorescence response to volatile acid vapors†
Abstract
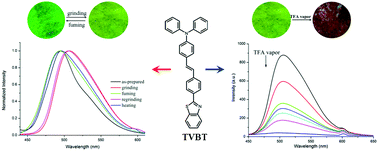
A novel D–π–A type triphenylamine modified benzothiazole derivative TVBT was synthesized, and it could emit strong fluorescence in solution and in the solid state. It should be noted that TVBT revealed reversible piezofluorochromic behaviors upon grinding and fuming with DCM vapor/heating treatment on account of the reversible transformation between the crystalline and amorphous states. The single crystal X-ray diffraction data revealed that the intermolecular interactions of C–H⋯N (2.54 Å, 2.68 Å), C–H⋯S (2.58 Å, 2.74 Å) and C–H⋯π (2.83 Å, 2.88 Å) would lead to the increased rigidity of the molecular skeleton, which would give rise to a loose packing motif, yielding piezofluorochromism. Interestingly, TVBT also exhibited reversible and remarkable acid/base-induced fluorescence switching properties in both solution and the solid state. In particular, the ground film of TVBT rapidly responded to TFA vapor. For example, the fluorescence gradually quenched with the increase of the concentration of TFA. When the concentration of TFA vapor was 459 ppm, the quenching efficiency reached 95.3%. In addition, the ground film of TVBT could also detect other volatile acid vapors, such as HCl and HNO3. Therefore, the ground film of TVBT could act as a fluorescence chemosensor with high performance for the naked-eye detection of volatile acid vapors by color and emission changes.

 Please wait while we load your content...
Please wait while we load your content...