Kinetic enhancement in high-activity enzyme complexes attached to nanoparticles†
Abstract
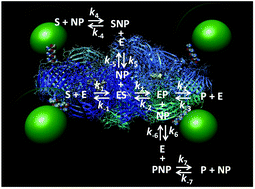
Accumulating studies by many groups have found consistent enhancement in a wide variety of enzyme activities when they are displayed around nanoparticles. However, the underlying mechanism(s) that give rise to this phenomenon are still largely unknown. Herein, we develop a detailed reaction scheme that considers many of the various possible interactions between a substrate and a given enzyme–nanoparticle bioconjugate. The properties and some functional predictions that emanate from the reaction scheme were then tested using a model system where the homotetrameric beta-galactosidase enzyme complex was assembled with luminescent semiconductor nanocrystalline quantum dots displayed around its periphery. This type of assembly occurs as the ∼465 kDa enzyme complex is significantly larger than the 4.2 nm diameter green emitting quantum dots utilized. This unique architecture, in conjunction with the fact that this enzyme functions at or near the diffusion limit, provided a unique opportunity to selectively probe certain aspects of enzyme enhancement when attached to a nanoparticle with minimal potential perturbations to the native enzyme structure. Experimental assays were conducted where both free enzymes and quantum dot-decorated enzymes were compared directly in side-by-side samples and included formats where the kinetic processes were challenged with increasing viscosity and competitive inhibitors. The results strongly suggest that it is possible for there to be significant enhancements in an enzyme's catalytic rate or kcat after attachment to a nanoparticle even when it is apparently diffusion limited without requiring any gross changes to the enzyme's structure. A discussion of how this reaction scheme and model can be applied to other systems is provided.


 Please wait while we load your content...
Please wait while we load your content...