Metal binding properties of zinc fingers with a naturally altered metal binding site†
Abstract
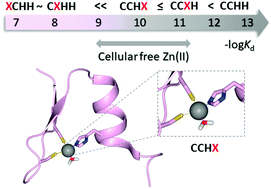
Zinc fingers (ZFs) are among the most abundant motifs found in proteins, and are commonly known for their structural role. Classical ZFs (CCHH) are part of the transcription factors that participate in DNA binding. Although biochemical studies of classical ZFs have a long history, there is limited knowledge about the sequential and structural diversity of ZFs. We have found that classical ZFs, with metal binding sites consisting of amino acids other than conserved Cys or His residues, are frequently encoded in the human genome, and we refer to these peptides as ZFs with a naturally altered metal binding site. The biological role of the altered ZFs remains undiscovered. In this study, we characterized nine natural XCHH, CXHH, CCXH and CCHX ZFs in terms of their Zn(II) and Co(II) binding properties, such as complex stoichiometry, spectroscopic properties and metal-to-peptide affinity. We revealed that XCHH and CXHH ZFs form ML complexes that are 4–5 orders of magnitude weaker in comparison to CCHH ZFs. Nevertheless, spectroscopic studies demonstrate that, depending on the altered position, they may adopt an open coordination geometry with one or two water molecules bound to a central metal ion, which has not been demonstrated in natural ZFs before. Stability data show that both CCXH and CCHX peptides have high Zn(II) affinity (with a Kd of 10−9 to 10−11 M), suggesting their potential biological function. This study is a comprehensive overview of the relationship between the sequence, structure, and stability of ZFs.


 Please wait while we load your content...
Please wait while we load your content...