Divalent metal ions control activity and inhibition of protein kinases
Abstract
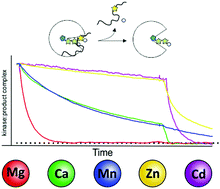
Protein kinases are key enzymes in the regulation of eukaryotic signal transduction. As metalloenzymes they employ divalent cations for catalysis and regulation. We used the catalytic (C) subunit of cAMP-dependent protein kinase (PKA) as a model protein to investigate the role of a variety of physiologically or pathophysiologically relevant divalent metal ions in distinct steps within the catalytic cycle. It is established that divalent metal ions play a crucial role in co-binding of nucleotides and also assist in catalysis. Our studies reveal that besides the physiologically highly relevant magnesium, metals like zinc and manganese can assist in phosphoryl transfer, however, only a few support efficient substrate turnover (turnover catalysis). Those trace metals allow for substrate binding and phosphotransfer but hamper product release. We further established the unique role of magnesium as the common biologically relevant divalent metal ideally enabling (co-) substrate binding and orientation. Magnesium allows stable substrate binding and, on the other hand accelerates product release, thus being extremely efficient in turnover catalysis. We extended our studies to non-catalytic functions of protein kinases looking at pseudokinases, a subfamily of protein kinases inherently lacking critical residues for catalysis. Recently, pseudokinases have been linked to human diseases. Some pseudokinases are still capable of binding metal ions, yet have lost the ability to transfer the phosphoryl group from ATP to a given substrate. Here metal ions stabilize an active like, though catalytically unproductive conformation and are therefore crucial to maintain non-catalytic function. Finally, we demonstrate for the canonical kinase PKA that the trace metal manganese alone can stabilize protein kinases in an active like conformation allowing them to bind substrates even in the absence of nucleotides.


 Please wait while we load your content...
Please wait while we load your content...