Rat ceruloplasmin: a new labile copper binding site and zinc/copper mosaic†
Abstract
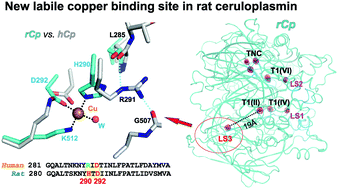
Ceruloplasmin (Cp) is a copper-containing multifunctional oxidase of plasma, an antioxidant, an acute-phase protein and a free radical scavenger. The structural organization of Cp causes its sensitivity to proteolysis and ROS (reactive oxygen species), which can alter some of the important Cp functions. Elucidation of the orthorhombic crystal structure of rat Cp at 2.3 Å resolution revealed the basis for stronger resistance of rat Cp to proteolysis and a new labile copper binding site. The presence of this site appears as a very rare and distinctive feature of rat Cp as was shown by sequence alignment of ceruloplasmin, hephaestin and zyklopen in the Deuterostomia taxonomic group. The trigonal crystal form of rat Cp at 3.2 Å demonstrates unexpected partial substitution of copper by zinc.


 Please wait while we load your content...
Please wait while we load your content...