Remarkable differences in the biochemical fate of Cd2+, Hg2+, CH3Hg+ and thimerosal in red blood cell lysate†
Abstract
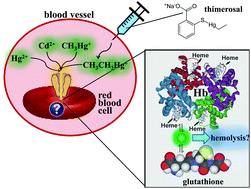
Humans are environmentally exposed to potentially toxic Cd and Hg species and to the Hg compound thimerosal (THI), an antibactericidal vaccine additive. Previous studies have revealed that Cd2+, Hg2+ and CH3Hg+ are taken up by red blood cells (RBCs) and bind to cytosolic glutathione (GSH) and/or hemoglobin (Hb). Since interactions in the cytosol of RBCs may be linked to their hemolysis, a more comprehensive characterization of these interactions was sought. After the addition of each Cd and Hg species to RBC lysate, the mixtures were analyzed after 5 min, 2 h and 6 h by size-exclusion chromatography (SEC) coupled on-line to an inductively coupled plasma atomic emission spectrometer (ICP-AES). In contrast to previous studies, however, reducing conditions were maintained by employing a 100 mM Tris buffer mobile phase (pH 7.4), which contained ∼2.5 mM of glutathione (GSH). At ≥2 h, ∼85% of Cd2+ weakly interacted with hemoglobin (Hb), while ∼13% eluted as (GS)xCd and ∼2% bound to a ≥70 kDa Cd-binding protein. In contrast, ∼6% of Hg2+ co-eluted with Hb at all time points, while ∼94% eluted as (GS)xHg. The results for CH3Hg+ showed that ∼5% of Hg co-eluted with Hb, while for THI this percentage gradually increased to 12% (6 h). The remaining Hg eluted as GS–HgCH3 and GS–HgCH2CH3. Our results revealed remarkable differences in the interaction of the investigated Cd and Hg species with cytosolic RBC constituents. The formation of (Hb)xHg species, regardless of which Hg compound was added, suggests their mammalian toxicology to be intertwined with the metabolism of Fe.

 Please wait while we load your content...
Please wait while we load your content...