Structure–function studies of acinetobactin analogs†
Abstract
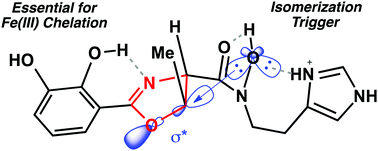
Pathogenic Acinetobacter baumannii excrete the siderophore pre-acinetobactin as an iron-scavenging virulence factor. Pre-acinetobactin is a 2,3-dihydroxy-phenyl oxazoline that undergoes pH-dependent isomerization to the isooxazolidinone form acinetobactin in order to expand the pH range for iron acquisition by A. baumannii. In this study we establish important structure–function relationships for the kinetics of isomerization, iron(III) binding, and siderophore utilization by A. baumannii. We showed that electronic properties of the phenyl oxazoline influence isomerization kinetics and iron(III) binding. We found that iron(III) chelation was directly correlated with A. baumannii utilization. Our studies provide important structural and mechanistic insight for understanding how pathogenic A. baumannii uses pre-acinetobactin as a 2-for-1 iron-scavenging siderophore virulence factor.
- This article is part of the themed collection: Metallomics Recent HOT articles


 Please wait while we load your content...
Please wait while we load your content...