The CO dehydrogenase accessory protein CooT is a novel nickel-binding protein†
Abstract
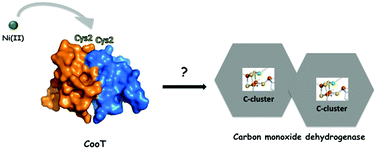
In Rhodospirillum rubrum, maturation of Carbon Monoxide Dehydrogenase (CODH) requires three accessory proteins, CooC, CooT and CooJ, dedicated to nickel insertion into the active site, which is constituted by a distorted [NiFe3S4] cubane coordinated with a mononuclear Fe site. CooC is an ATPase proposed to provide the energy required for the maturation process, while CooJ is described as a metallochaperone with 16 histidines and 2 cysteines at the C-terminus, likely involved in metal binding and/or storage. Prior to the present study, no information was available on CooT at the molecular level. Here, the X-ray structure of RrCooT was obtained, which revealed that this protein is a homodimer featuring a fold that resembles an Sm-like domain, suggesting a role in RNA metabolism that was however not supported by experimental observations. Biochemical and biophysical evidence based on circular dichroism spectroscopy, light scattering, isothermal titration calorimetry and site-directed mutagenesis showed that RrCooT specifically binds a single Ni(II) per dimer, with a dissociation constant of 9 nM, through the pair of Cys2, highly conserved residues, located at the dimer interface. Despite its role in the activation of RrCODH in vivo, CooT was thought to be a unique protein, found only in R. rubrum, with an unclear function. In this study, we extended the biological impact of CooT, establishing that this protein is a member of a novel Ni(II)-binding protein family with 111 homologues, linked to anaerobic metabolism in bacteria and archaea, and in most cases to the presence of CODH.
- This article is part of the themed collection: Nickel in biology


 Please wait while we load your content...
Please wait while we load your content...