Tight binding of heme to Staphylococcus aureus IsdG and IsdI precludes design of a competitive inhibitor†
Abstract
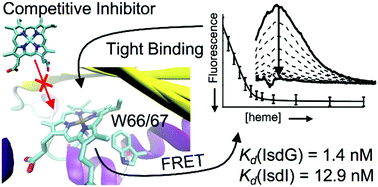
The micromolar equilibrium constants for heme dissociation from IsdG and IsdI reported in the literature call into question whether these enzymes are actually members of the iron-regulated surface determinant system of Staphylococcus aureus, which harvests heme iron from a host during infection. In order to address this question, the heme dissociation constants for IsdG and IsdI were reevaluated using three approaches. The heme dissociation equilibrium constants were measured using a UV/Vis absorption-detected assay analyzed with an assumption-free model, and using a newly developed fluorescence-detected assay. The heme dissociation rate constants were estimated using apomyoglobin competition assays. Analyses of the UV/Vis absorption data revealed a critical flaw in the previous measurements; heme is 99.9% protein-bound at the micromolar concentrations needed for UV/Vis absorption spectroscopy, which renders accurate equilibrium constant measurement nearly impossible. However, fluorescence can be measured for more dilute samples, and analyses of these data resulted in dissociation equilibrium constants of 1.4 ± 0.6 nM and 12.9 ± 1.3 nM for IsdG and IsdI, respectively. Analyses of the kinetic data obtained from apomyoglobin competition assays estimated heme dissociation rate constants of 0.022 ± 0.002 s−1 for IsdG and 0.092 ± 0.008 s−1 for IsdI. Based upon these data, and what is known regarding the post-translational regulation of IsdG and IsdI, it is proposed that only IsdG is a member of the heme iron acquisition pathway and IsdI regulates heme homeostasis. Furthermore, the nanomolar dissociation constants mean that heme is bound tightly by IsdG and indicates that competitive inhibition of this protein will be difficult. Instead, uncompetitive inhibition based upon a detailed understanding of enzyme mechanism is a more promising antibiotic development strategy.
- This article is part of the themed collection: Metallomics 2017 Most Downloaded Articles


 Please wait while we load your content...
Please wait while we load your content...