Na/K-ATPase as a target for anticancer metal based drugs: insights into molecular interactions with selected gold(iii) complexes†
Abstract
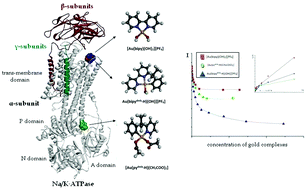
Na/K-ATPase is emerging as an important target for a variety of anticancer metal-based drugs. The interactions of Na/K-ATPase (in its E1 state) with three representative and structurally related cytotoxic gold(III) complexes, i.e. [Au(bipy)(OH)2][PF6], bipy = 2,2′-bipyridine; [Au(pydmb-H)(CH3COO)2], pydmb-H = deprotonated 6-(1,1-dimethylbenzyl)-pyridine and [Au(bipydmb-H)(OH)][PF6], bipyc-H = deprotonated 6-(1,1-dimethylbenzyl)-2,2′-bipyridine, are investigated here in depth using a variety of spectroscopic methods, in combination with docking studies. Detailed information is gained on the conformational and structural changes experienced by the enzyme upon binding of these gold(III) complexes. The quenching constants of intrinsic enzyme fluorescence, the fraction of Trp residues accessible to gold(III) complexes and the reaction stoichiometries were determined in various cases. Specific hypotheses are made concerning the binding mode of these gold(III) complexes to the enzyme and the likely binding sites. Differences in their binding behaviour toward Na/K-ATPase are explained on the ground of their distinctive structural features. The present results offer further support to the view that Na/K-ATPase may be a relevant biomolecular target for cytotoxic gold(III) compounds of medicinal interest and may thus be involved in their overall mode of action.


 Please wait while we load your content...
Please wait while we load your content...