Copper binding and redox chemistry of the Aβ16 peptide and its variants: insights into determinants of copper-dependent reactivity†
Abstract
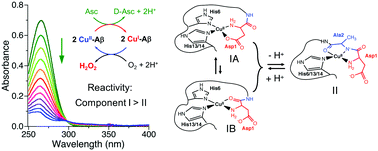
The metal-binding sites of Aβ peptides are dictated primarily by the coordination preferences of the metal ion. Consequently, Cu(I) is typically bound with two His ligands in a linear mode while Cu(II) forms a pseudo-square planar stereochemistry with the N-terminal amine nitrogen acting as an anchoring ligand. Several distinct combinations of other groups can act as co-ligands for Cu(II). A population of multiple binding modes is possible with the equilibrium position shifting sensitively with solution pH and the nature of the residues in the N-terminal region. This work examined the Cu(II) chemistry of the Aβ16 peptide and several variants that targeted these binding modes. The results are consistent with: (i) at pH < 7.8, the square planar site in CuII–Aβ16 consists primarily of a bidentate ligand provided by the carboxylate sidechain of Asp1 and the N-terminal amine supported by the imidazole sidechains of two His residues (designated here as component IA); it is in equilibrium with a less stable component IB in which the carboxylate ligand is substituted by the Asp1-Ala2 carbonyl oxygen. (ii) Both IA and IB convert to a common component II (apparent transition pKa ∼7.8 for IA and ∼6.5 for IB, respectively) featuring a tridentate ligand consisting of the N-terminal amine, the Asp1-Ala2 amide and the Ala2-Pro3 carbonyl; this stereochemistry is stabilized by two five-membered chelate rings. (iii) Component IA is stabilized for variant Aβ16-D1H, components I (both IA and IB) are imposed on Aβ16-A2P while the less stable IB is enforced on Aβ16-D1A (which is converted to component II at pH ∼6.5); (iv) components IA and IB share two His ligands with Cu(I) and are more reactive in redox catalysis than component II that features a highly covalent and less reactive amide N− ligand. The redox activity of IA is further enhanced for peptides with a His1 N-terminus that may act as a ligand for either Cu(I) or Cu(II) with lower re-organization energy required for redox-shuttling. This study provided insights into the determinants that regulate the reactivity of Cu–Aβ complexes.


 Please wait while we load your content...
Please wait while we load your content...