Oxygen-dependent activation of Cu,Zn-superoxide dismutase-1
Abstract
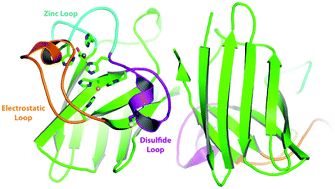
Copper zinc superoxide dismutase (Sod1) is a critical enzyme in limiting reactive oxygen species in both the cytosol and the mitochondrial intermembrane space. Sod1 dismutes superoxide anions to hydrogen peroxide and oxygen. The catalytic reaction is dependent on an active site copper ion and a disulfide bonded conformation. The activation of Sod1 is mediated by its chaperone Ccs1. The mechanism of Ccs1-mediated Sod1 activation involves both insertion of the catalytic copper ion and mediating disulfide bond formation. Since Sod1 is a highly abundant enzyme residing within the highly reducing cytoplasm, the question of disulfide bond formation is significant yet unresolved. The processes involved in Sod1 activation are reviewed with a focus on copper ion insertion and disulfide bond formation.
- This article is part of the themed collection: Recent Review Articles

 Please wait while we load your content...
Please wait while we load your content...