Phosphorylation of zinc channel ZIP7 drives MAPK, PI3K and mTOR growth and proliferation signalling†
Abstract
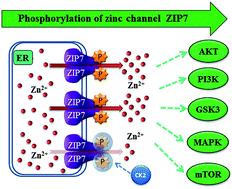
Zinc is an essential trace element participating in diverse biological processes. Cellular zinc levels are strictly controlled by two families of transport proteins: ZIP channels (SLC39A) and ZnT transporters (SLC30A). ZIP channels increase cytosolic zinc levels by importing zinc into cells or releasing zinc from intracellular stores such as the ER. Among all the 14 human members of the ZIP family, ZIP7 is a gatekeeper of zinc release from intracellular stores, requiring post-translational activation by phosphorylation on residues S275 and S276, resulting in activation of multiple downstream pathways. Employing site-directed mutagenesis, we investigated the importance of these individual serine residues as well as other predicted phosphorylation sites on ZIP7, showing that all four sites are required for maximal ZIP7 activation. Using phosphor-protein arrays, we also discovered the major signalling pathways that were activated as a direct result of ZIP7-mediated zinc release from intracellular stores. These data reveal the role of ZIP7-mediated zinc release from intracellular stores in driving major pathways, such as MAPK, mTOR and PI3K-AKT, involved in providing cell survival and proliferation and often over activated in cancer.
- This article is part of the themed collection: Metallomics 2017 Most Downloaded Articles


 Please wait while we load your content...
Please wait while we load your content...