Biochemical characterization of the selenoproteome in Gallus gallus via bioinformatics analysis: structure–function relationships and interactions of binding molecules†
Abstract
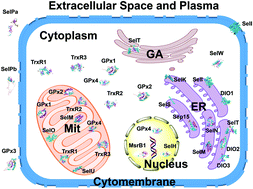
Knowledge about mammalian selenoproteins is increasing. However, the selenoproteome of birds remains considerably less understood, especially concerning its biochemical characterization, structure–function relationships and the interactions of binding molecules. In this work, the SECIS elements, subcellular localization, protein domains and interactions of binding molecules of the selenoproteome in Gallus gallus were analyzed using bioinformatics tools. We carried out comprehensive analyses of the structure–function relationships and interactions of the binding molecules of selenoproteins, to provide biochemical characterization of the selenoproteome in Gallus gallus. Our data provided a wealth of information on the biochemical functions of bird selenoproteins. Members of the selenoproteome were found to be involved in various biological processes in chickens, such as in antioxidants, maintenance of the redox balance, Se transport, and interactions with metals. Six membrane-bound selenoproteins (SelI, SelK, SelS, SelT, DIO1 and DIO3) played important roles in maintaining the membrane integrity. Chicken selenoproteins were classified according to their ligand binding sites as zinc-containing matrix metalloselenoproteins (Sep15, MsrB1, SelW and SelM), POP-containing selenoproteins (GPx1–4), FAD-interacting selenoproteins (TrxR1–3), secretory transport selenoproteins (GPx3 and SelPa) and other selenoproteins. The results of our study provided new evidence for the unknown biological functions of the selenoproteome in birds. Future research is required to confirm the novel biochemical functions of bird selenoproteins.
- This article is part of the themed collection: Metallomics Recent HOT articles


 Please wait while we load your content...
Please wait while we load your content...