Detection of Zn2+ release in nitric oxide treated cells and proteome: dependence on fluorescent sensor and proteomic sulfhydryl groups
Abstract
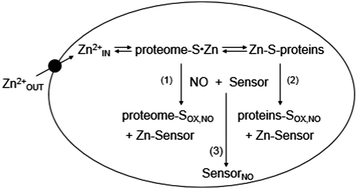
Nitric oxide (NO) is both an important regulatory molecule in biological systems and a toxic xenobiotic. Its oxidation products react with sulfhydryl groups and either nitrosylate or oxidize them. The aerobic reaction of NO supplied by diethylamine NONOate (DEA-NO) with pig kidney LLC-PK1 cells and Zn-proteins within the isolated proteome was examined with three fluorescent zinc sensors, zinquin (ZQ), TSQ, and FluoZin-3 (FZ-3). Observations of Zn2+ labilization from Zn-proteins depended on the specific sensor used. Upon cellular exposure to DEA-NO, ZQ sequestered about 13% of the proteomic Zn2+ as Zn(ZQ)2 and additional Zn2+ as proteome·Zn–ZQ ternary complexes. TSQ, a sensor structurally related to ZQ with lower affinity for Zn2+, did not form Zn(TSQ)2. Instead, Zn2+ mobilized by DEA-NO was exclusively bound as proteome·Zn–TSQ adducts. Analogous reactions of proteome with ZQ or TSQ in vitro displayed qualitatively similar products. Titration of native proteome with Zn2+ in the presence of ZQ resulted in the sole formation of proteome·Zn–ZQ species. This result suggested that sulfhydryl groups are involved in non-specific proteomic binding of mobile Zn2+ and that the appearance of Zn(ZQ)2 after exposure of cells and proteome to DEA-NO resulted from a reduction in proteomic sulfhydryl ligands, favoring the formation of Zn(ZQ)2 instead of proteome·Zn–ZQ. With the third sensor, FluoZin-3, neither Zn–FZ-3 nor proteome·Zn–FZ-3 was detected during the reaction of proteome with DEA-NO. Instead, it reacted independently with DEA-NO with a modest enhancement of fluorescence.
- This article is part of the themed collections: Zinc in the Biosciences and Imaging Metals in Biology

 Please wait while we load your content...
Please wait while we load your content...