Effective design of supramolecular polymer adhesives based on multiple CH/π interactions†
Abstract
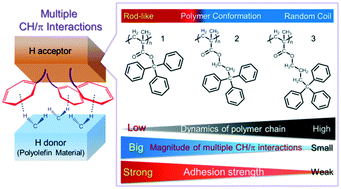
Adhesion to chemically inert materials without surface modification through noncovalent interactions represents a challenging task in materials science and industry. Herein, we report the effective structural design of a poly(methacrylate) bearing aromatic rings in its side chains (H acceptor) that can adhere to chemically inert polyolefin (H donor) materials through multiple CH/π interactions. We designed and compared adhesives based on different H acceptors, i.e., poly(trityl methacrylate) 1, having a rigid rod-like structure; poly(3,3,3-triphenyl-1-propyl methacrylate) 2; and poly(4,4,4-triphenyl-1-butyl methacrylate) 3, exhibiting random coil structures and different kinematic polymer chain characteristics. The observed strength of adhesion to polyolefins increased with decreasing mobility of the H-acceptor polymer chain, supporting the results of interfacial analyses based on experimental and theoretical approaches. These findings suggest that restricting the molecular motion of the H-acceptor effectively promotes multiple CH/π interactions with polyolefins. We conclude that rigid, rod-like polymers containing multiple aromatic rings in their side chains are favourable H acceptors for the structural design of our adhesion system. We expect our study to aid the further development of new adhesives for chemically inert materials such as polyolefins, paving the way for the design of novel adhesion systems utilizing weak non-covalent interactions.


 Please wait while we load your content...
Please wait while we load your content...