Evaluation of de novo-designed coiled coils as off-the-shelf components for protein assembly†
Abstract
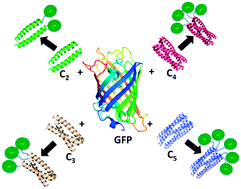
Coiled-coil domains are attractive modular components for assembling individual protein subunits into higher order structures because they can be designed de novo with well-defined oligomerization states, topologies, and dissociation energies. However, the utility of coiled-coil designs as plug-and-play components for synthetic biology applications depends critically on them robustly maintaining their oligomerization states when fused to larger proteins of interest. Here, we investigate the ability of a series of well-characterized de novo-designed parallel coiled coils, with oligomerization states ranging from dimer to pentamer, to mediate the oligomerization of a model monomeric protein, green fluorescent protein (GFP). Six coiled-coil GFP fusion proteins were initially constructed and their oligomerization states investigated using size exclusion chromatography, analytical ultracentrifugation, and native mass spectrometry. Somewhat surprisingly, only two of these initial designs adopted their intended oligomerization states. However, with minor refinements, the intended oligomerization states of two of the four other constructs could be achieved. Parameters found to influence the oligomerization state of the GFP fusions included the number of heptad repeats and the length of the linker sequence separating GFP from the coiled coil. These results demonstrate that even for stable, well-designed coiled coils, the oligomerization state is subject to unanticipated changes when connected to larger protein components. Therefore, although coiled coils can be successfully used as components in protein designs their ability to achieve the desired oligomerization state requires experimental verification.


 Please wait while we load your content...
Please wait while we load your content...