Design, synthesis and biological evaluation of tacrine-1,2,3-triazole derivatives as potent cholinesterase inhibitors†
Abstract
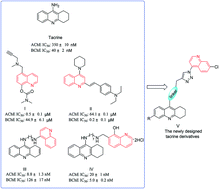
We report herein the design and synthesis of a series of 11 novel tacrine-1,2,3-triazole derivatives via a Cu(I)-catalyzed alkyne–azide 1,3-dipolar cycloaddition (CuAAC) reaction. The newly synthesized compounds were evaluated for their inhibition activity against Electrophorus electricus acetylcholinesterase (AChE) and horse serum butyrylcholinesterase (BChE) as potential drug targets for Alzheimer's disease (AD). Among the designed compounds, compound 8a2 exhibited potent inhibition against AChE and BChE with IC50 values of 4.89 μM and 3.61 μM, respectively. Further structure–activity relationship (SAR) and molecular modeling studies may provide valuable insights into the design of better tacrine-triazole analogues with potential therapeutic applications for AD.


 Please wait while we load your content...
Please wait while we load your content...