Development of NMR and thermal shift assays for the evaluation of Mycobacterium tuberculosis isocitrate lyase inhibitors†
Abstract
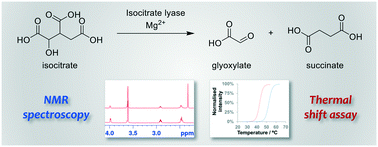
The enzymes isocitrate lyase (ICL) isoforms 1 and 2 are essential for Mycobacterium tuberculosis survival within macrophages during latent tuberculosis (TB). As such, ICLs are attractive therapeutic targets for the treatment of tuberculosis. However, there are few biophysical assays that are available for accurate kinetic and inhibition studies of ICL in vitro. Herein we report the development of a combined NMR spectroscopy and thermal shift assay to study ICL inhibitors for both screening and inhibition constant (IC50) measurement. Operating this new assay in tandem with virtual high-throughput screening has led to the discovery of several new ICL1 inhibitors.


 Please wait while we load your content...
Please wait while we load your content...