Cu(ii), Ga(iii) and In(iii) complexes of 2-acetylpyridine N(4)-phenylthiosemicarbazone: synthesis, spectral characterization and biological activities†
Abstract
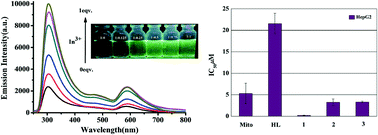
In this paper, synthesis and characterization of metal complexes [Cu2(L)3]ClO4 (1), [Ga(L)2]NO3·2H2O (2) and [In(L)2]NO3·H2O (3) (HL = 2-acetylpyridine N(4)-phenylthiosemicarbazone) was carried out, including elemental analysis, spectral analysis (IR, UV-vis, NMR), and X-ray crystallography. Complex 1 contains one S-bridged binuclear [Cu2(L)3]+ unit, where two Cu atoms display diverse coordination geometries: one being square planar geometry and the other octahedral geometry. Both 2 and 3 are mononuclear complexes, and the metal centers in 2 and 3 are chelated by two NNS tridentate ligands possessing a distorted octahedral geometry. Biological studies show that all the complexes possess a wide spectrum of modest to effective antibacterial activities and remarkable cytotoxicities against HepG2 cells, and 1, in particular, with an IC50 value of 0.19 ± 0.06 μM, is 113-fold and 28-fold more cytotoxic than HL and the antitumor drug mitoxantrone, respectively. In addition, 3 exhibits excellent photoluminescence properties. Upon the addition of 1 equiv of In3+ ions, a remarkable fluorescence intensity of HL and fluorescent color change (from transparent to light-green) could be observed with 365 nm light, indicating that this ligand may be used as a promising colorimetric and fluorescent probe for In3+ detection.


 Please wait while we load your content...
Please wait while we load your content...