A mini-library system to investigate non-essential residues of lipid-phosphatidylserine (PS) binding peptide–peptoid hybrid PPS1†
Abstract
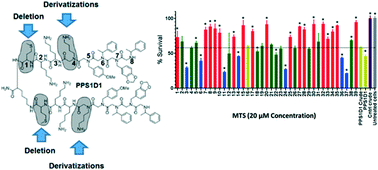
We recently identified a peptide–peptoid hybrid, PPS1, which specifically recognized lipid-phosphatidylserine (PS). PPS1 consists of distinct positively charged and hydrophobic residue-containing regions. The PPS1 monomer was inactive, but the dimeric form, PPS1D1, displayed strong cytotoxicity to lung cancer cells compared to normal cells in vitro, and reduced the tumor growth in vivo. The minimum pharmacophore of PPS1D1 showed that the first (methionine) and fourth (N-lysine) residues were not important for PPS1D1 cytotoxic activity. In this study, we further investigated these two residues, in particular the fourth residue that lies between the most important four-residue hydrophobic region and two positively charged residues, to determine whether replacements of these moieties could gain activity improvements or render PPS1D1 totally insensitive for binding recognition. The positively charged fourth residue N-lysine was replaced with substituents having varied physiochemical properties, such as aromatic-hydrophobic, aliphatic-alicyclic, heterocyclic, and negatively charged residues, developing a mini-library of 39 derivatives. The standard 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) colorimetric and/or the calcein AM cell viability assays performed on HCC4017 lung cancer cells indicated that the fourth position of PPS1D1 was insensitive to most changes, except that reversal of the negative charge significantly affected the activity. This observation may be due to the neutralization of the nearby positively charged residue that is essential for binding. In addition, shortening each monomeric sequence by eliminating the methionine at the first position did not affect the activity.


 Please wait while we load your content...
Please wait while we load your content...