One-pot synthesis, anti-tumor evaluation and structure–activity relationships of novel 25-OCH3-PPD derivatives†
Abstract
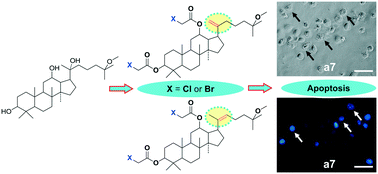
Based on the fact that 25-OCH3-PPD, a natural ginsengenin isolated from the leaves of Panax ginseng, is a promising lead compound, novel 25-OCH3-PPD derivatives were synthesized to find more potent anti-tumor agents by a simple and facile synthetic method. These derivatives were classified into three types and screened for their cytotoxic activities against seven human cancer cell lines. Compared with 25-OCH3-PPD, compounds a5, a7, b5 and b7 exhibited higher anti-tumor activities on all tested cell lines with almost 5-fold to 15-fold increases. In particular, compound a7 showed the greatest cytotoxic activity against α-2 cells (IC50 = 2.4 ± 0.4 μM). The preliminary study on the mechanisms indicated that compound a7 could induce α-2 cell apoptosis. Structure–activity relationships demonstrated that the carbon–carbon double bond at the C-20 position could enhance the antiproliferative activity. In conclusion, the novel derivatives a5, a7, b5 and b7 could be further studied as potential candidates for the treatment of cancer. This research provides a theoretical reference for the exploration of new antiproliferative agents.

 Please wait while we load your content...
Please wait while we load your content...