Cu(ii) immobilized on Fe3O4@APTMS-DFX nanoparticles: an efficient catalyst for the synthesis of 5-substituted 1H-tetrazoles with cytotoxic activity
Abstract
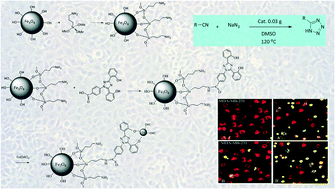
Cu(II) immobilized on deferasirox loaded amine functionalized magnetic nanoparticles (Cu(II)/Fe3O4@APTMS-DFX) as a novel magnetically recyclable heterogeneous catalyst is able to catalyze the [3 + 2] cycloaddition reactions of various organic nitriles with sodium azide. Using this method, a series of 5-substituted-1H-tetrazoles under mild conditions in DMSO were prepared. The reaction involves mild reaction conditions with efficient transformation capability. The developed catalyst could be easily separated by applying an external magnetic field. Furthermore, it could be recycled for 5 runs with negligible leaching of copper from the surface of the catalyst. The catalyst was characterized by various techniques such as FT-IR, TGA, VSM, SEM-EDX, and ICP-OES. Several derivatives of 1H-tetrazoles were prepared using this catalyst, and their structures were confirmed using different techniques. Then, the synthesized anthraquinones were evaluated for their cytotoxicity against several cell lines including MCF-7, MAD-MD-231, HT-29, HeLa, neuro-2a and L-929. The results obtained from the MTT assay revealed that the 6 derivatives exhibited a high level of cytotoxicity. In order to determine the cytotoxicity mechanism, 2 derivatives with the highest cytotoxic activity were selected, and an apoptosis assay was carried out by flow cytometry, which supported that apoptosis is the major mechanism.


 Please wait while we load your content...
Please wait while we load your content...