Bioisosteric replacement of central 1,2,4-oxadiazole ring of high affinity CB2 ligands by regioisomeric 1,3,4-oxadiazole ring†
Abstract
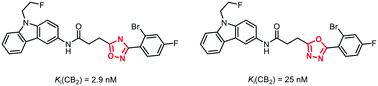
It has been reported that bioisosteric replacement of an 1,2,4-oxadiazole ring by an 1,3,4-oxadiazole ring leads to higher polarity, reduced metabolic degradation by human liver microsomes and reduced interaction with hERG channels. In a seven to eight step synthesis 1,3,4-oxadiazles 9a–c were synthesized as bioisosteric analogs of high-affinity but rather lipophilic CB2 ligands 1a–c containing an 1,2,4-oxadiazole ring. The 1,3,4-oxadiazole derivatives 9a and 9b show 10- and 50-fold reduced CB2 affinity compared to the 1,2,4-oxadiazole derivatives 1a and 1b, respectively. However, the 1,3,4-oxadiazole 9a has high CB2 affinity (Ki = 25 nM) and high selectivity over the CB1 receptor.


 Please wait while we load your content...
Please wait while we load your content...