Development of selective agents targeting serotonin 5HT1A receptors with subnanomolar activities based on a coumarin core†
Abstract
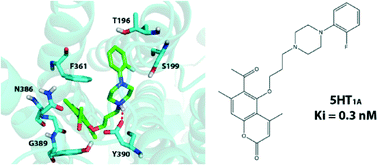
A series of 18 new 5-[3-(4-aryl-1-piperazinyl)propoxy]coumarin derivatives from the corresponding bromoalkyl derivatives have been designed and synthesized by us using a microwave-assisted protocol. Radioligand binding assays of this series of compounds as well as a previously synthesized series of 17 structurally-similar compounds showed that six systems have very high affinities to the 5-HT1A receptor (0.3–1.0 nM) and good selectivity against the 5-HT2A receptor. Molecular docking, structural studies and structure–activity relationship studies were used to gain more insight into the atomistic details of ligand binding and rationalize the obtained results.


 Please wait while we load your content...
Please wait while we load your content...