Synthesis and evaluation of oxindoles as promising inhibitors of the immunosuppressive enzyme indoleamine 2,3-dioxygenase 1†
Abstract
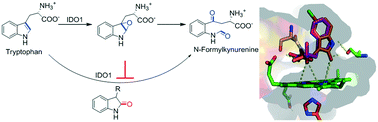
Indoleamine 2,3-dioxygenase 1 (IDO1) is considered as an important therapeutic target for the treatment of cancer, chronic infections and other diseases that are associated with immune suppression. Recent developments in understanding the catalytic mechanism of the IDO1 enzyme revealed that conversion of L-tryptophan (L-Trp) to N-formylkynurenine proceeded through an epoxide intermediate state. Accordingly, we synthesized a series of 3-substituted oxindoles from L-Trp, tryptamine and isatin. Compounds with C3-substituted oxindole moieties showed moderate inhibitory activity against the purified human IDO1 enzyme. Their optimization led to the identification of potent compounds, 6, 22, 23 and 25 (IC50 = 0.19 to 0.62 μM), which are competitive inhibitors of IDO1 with respect to L-Trp. These potent compounds also showed IDO1 inhibition potencies in the low-micromolar range (IC50 = 0.33–0.49 μM) in MDA-MB-231 cells. The cytotoxicity of these potent compounds was trivial in different model cancer (MDA-MB-231, A549 and HeLa) cells and macrophage (J774A.1) cells. Stronger selectivity for the IDO1 enzyme (124 to 210-fold) over the tryptophan 2,3-dioxygenase (TDO) enzyme was also observed for these compounds. These results suggest that the oxindole moiety of the compounds could mimic the epoxide intermediate state of L-Trp. Therefore, the structural simplicity and low-micromolar inhibition potencies of these 3-substituted oxindoles make them quite attractive for further investigation of IDO1 function and immunotherapeutic applications.


 Please wait while we load your content...
Please wait while we load your content...