Tetrahydroquinolinone derivatives as potent P-glycoprotein inhibitors: design, synthesis, biological evaluation and molecular docking analysis†
Abstract
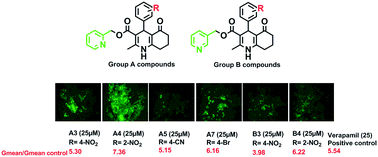
P-glycoprotein (P-gp) is a transmembrane efflux pump that has been associated with ineffective cancer chemotherapy and multidrug resistance (MDR). Chemical inhibitors of P-gp could have potential cancer therapeutic applications by preventing or reversing MDR. To exploit this, we designed twenty-five tetrahydroquinolinone analogs bearing pyridyl methyl carboxylate at C3 and different substituents at C4 as MDR reversal agents. The inhibitory effects of the synthesized compounds against P-gp were assessed by flow cytometric determination of rhodamine 123 accumulation in P-gp over-expressing MES-SA/DX5 cells. Fluorescence imaging of intracellular rhodamine 123 accumulation in MES-SA/DX5 cells was also performed. Furthermore, the effect of active derivatives on the reduction of doxorubicin's IC50 in MES-SA/DX5 cells was evaluated using MTT assay. Molecular docking was used to confirm the binding mode of some of the synthesized compounds. Five compounds in group A, bearing a 2-pyridyl methyl ester substituent at the C3 position, significantly increased rhodamine accumulation at 25 μM comparable to verapamil, a well-established P-gp inhibitor, while only 2 compounds in group B bearing 3-pyridyl methyl ester at the same position had this effect. This study shows that tetrahydroquinolinones containing methyl pyridine esters could represent an attractive scaffold for the discovery of P-gp inhibitors as MDR reversal agents in cancer cells.


 Please wait while we load your content...
Please wait while we load your content...