Synthesis, molecular modeling and biological evaluation of aza-flavanones as α-glucosidase inhibitors†
Abstract
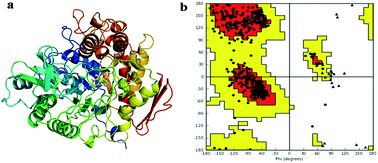
An efficient acid catalyzed methodology has been employed to synthesize a variety of aza-flavanones and their α-glucosidase inhibitory activity is evaluated using acarbose, miglitol and voglibose as reference standards. Molecular modeling studies were performed for all compounds to identify the important binding modes responsible for the inhibition activity of α-glucosidase which helped find key interactions between the enzyme and the active compounds. Among all the compounds 5g, 5r and 5w have shown high α-glucosidase inhibition activity compared to standard reference drugs and have been identified as promising potential antidiabetic agents. This study is the first biological evaluation of aza-flavanones as α-glucosidase inhibitors.


 Please wait while we load your content...
Please wait while we load your content...