An insight into tetrahydro-β-carboline–tetrazole hybrids: synthesis and bioevaluation as potent antileishmanial agents†
Abstract
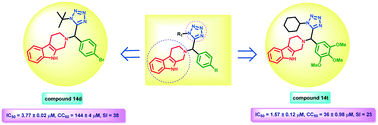
A series of 2,3,4,9-tetrahydro-β-carboline tetrazole derivatives (14a–u) have been synthesized utilizing the Ugi multicomponent reaction and were identified as potential antileishmanial chemotypes. Most of the screened derivatives exhibited significant in vitro activity against the promastigote (IC50 from 0.59 ± 0.35 to 31 ± 1.27 μM) and intracellular amastigote forms (IC50 from 1.57 ± 0.12 to 17.6 ± 0.2 μM) of L. donovani, and their activity is comparable with standard drugs miltefosine and sodium stibogluconate. The most active compound 14t was further studied in vivo against the L. donovani/golden hamster model at a dose of 50 mg kg−1 through the intraperitoneal route for 5 consecutive days, which displayed 75.04 ± 7.28% inhibition of splenic parasite burden. Pharmacokinetics of compound 14t was studied in the golden Syrian hamster, and following a 50 mg kg−1 oral dose, the compound was detected in hamster serum for up to 24 h. It exhibited a large volume of distribution (651.8 L kg−1), high clearance (43.2 L h−1 kg−1) and long mean residence time (10 h).


 Please wait while we load your content...
Please wait while we load your content...